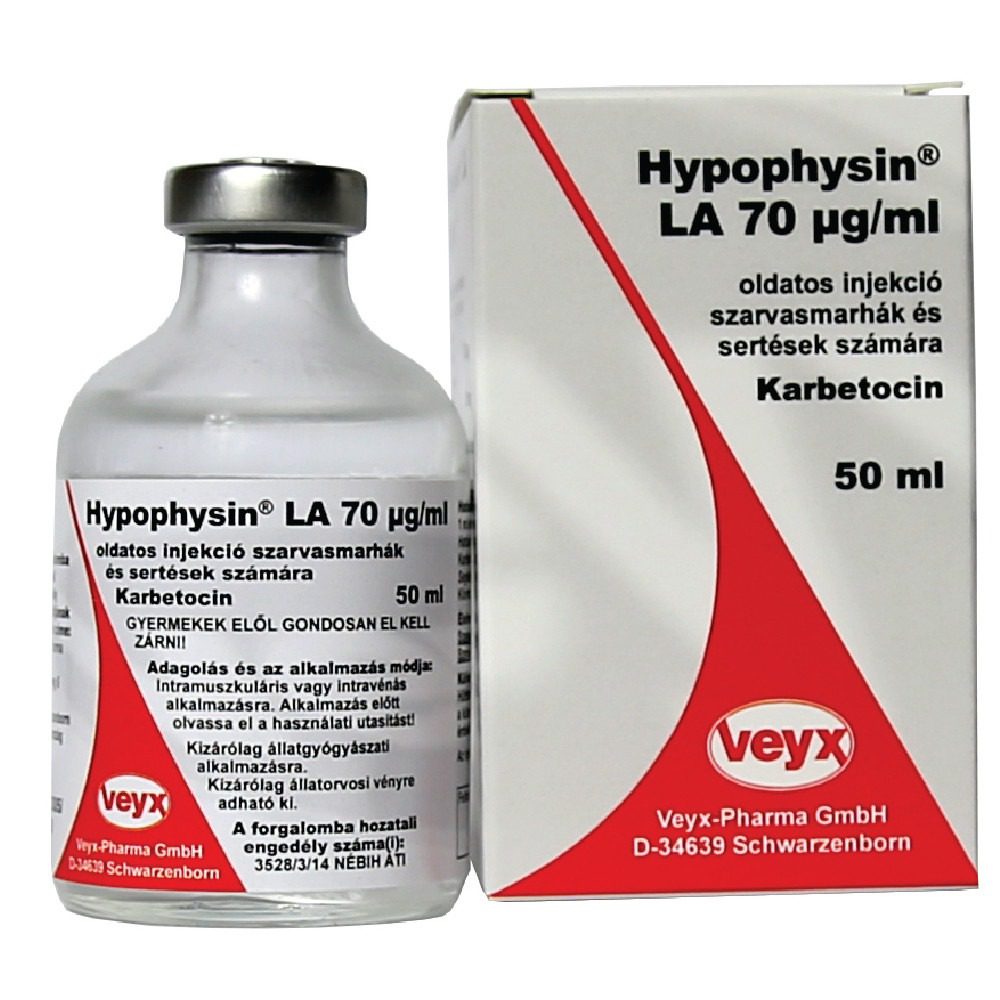
Hypophysin LA
PLEASE NOTE: A prescription issued by a UK registered vet (MRCVS) is required before this item can be dispatched.

Hypophysin LA 50ml
Hypophysin LA is a long-acting injectable solution designed to support reproductive health and aid in the management of parturition in livestock. Containing oxytocin, a naturally occurring hormone, Hypophysin LA stimulates uterine contractions to facilitate calving or lambing and assists in the expulsion of the placenta. It’s also effective in promoting milk let-down, making it essential for use in cases of difficult births or lactation issues in cattle, sheep, and pigs.
Key Features:
- Oxytocin-Based Formula: Contains oxytocin, a hormone that stimulates uterine contractions and aids in parturition and milk release.
- Facilitates Calving & Lambing: Helps in difficult births by encouraging smooth uterine contractions, ensuring a safer delivery for both the mother and offspring.
- Supports Milk Let-Down: Assists in triggering milk release in lactating animals, helping with issues of delayed or insufficient milk production.
- Long-Acting: Provides extended efficacy, reducing the need for frequent re-administration and ensuring sustained support during critical periods.
- Versatile Use: Suitable for use in cattle, sheep, and pigs, making it a versatile option for livestock reproductive management.
Benefits:
- Eases Parturition: Helps reduce complications during calving or lambing by promoting timely and effective uterine contractions.
- Improves Post-Birth Recovery: Aids in the expulsion of the placenta, reducing the risk of post-parturition complications and promoting faster recovery.
- Promotes Lactation: Effectively stimulates milk let-down, ensuring adequate milk supply for newborn animals and improving lactation management.
- Trusted by Veterinarians: Widely recommended for its efficacy and reliability in reproductive health management and lactation support.
Hypophysin LA is an essential tool for farmers and veterinarians, providing vital support during parturition and lactation challenges in livestock. It ensures safer births and better lactation, contributing to overall herd health and productivity.
Target species Cattle, pigs 4.2 Indications for use, specifying the target species Cows: – Uterine atony during the puerperal period – Placental retention as a consequence of uterine atony – Initiation of milk ejection in stress-induced agalactia or in conditions requiring udder emptying
Sows: – Acceleration or restart of parturition after disruption of uterine contractions (uterine atony or inertia) following the expulsion of at least one piglet – Supportive therapy of mastitis-metritis-agalactia (MMA-) syndrome – Initiation of milk ejection – Shortening of total parturition duration as a component of synchronisation of parturition in sows The product may be applied to sows which have previously been administered an appropriate analogue (e.g. cloprostenol) not prior to day 114 of pregnancy and have not started farrowing within 24 hours after the PGF2 or PGF2 analogue injection (day 1 of pregnancy is the last day of insemination) 4.3
Contraindications Do not administer to accelerate parturition if cervix is not opened or if there is a mechanical cause for the delayed parturition such as physical obstruction, positional and postural abnormalities, convulsive labour, threatened rupture of uterus, uterine torsion, relative foetal oversize or deformities of the birth canal. Do not use in cases of hypersensitivity to the active substance or to any of the excipients. 4.4
Special warnings for each target species The responsiveness to carbetocin of the myometrium is likely to be close to zero from the 5th to the 11th day post-partum. Therefore, the administration of the veterinary medicinal product during this period is likely to be inefficient and should be avoided. If treatment with carbetocin should fail, then it is advisable to reconsider the aetiology of the condition, specifically if hypocalcaemia could be a complicating factor. In case of severe septic metritis, appropriate concomitant therapy should be instigated when administering the veterinary medicinal product. 4.5
Special precautions for use in animals None.
Special precautions to be taken by the person administering the veterinary medici-nal product to animals In case of accidental self-injection uterine contractions could be induced in pregnant women. Pregnant women, women post-partum and breast-feeding women should not ad-minister this product, in order to avoid an accidental exposure. In case of an accidental self-injection of the veterinary medicinal product in non-pregnant women the following effects may occur: facial flushing and warmth, lower abdominal pain. These effects usually disappear within a short span of time. In the case of accidental self-injection, seek medical advice immediately and show the package leaflet or the label to the physician. Personal protective equipment consisting of disposable gloves should be worn when handling the veterinary medicinal product. Carbetocin may be absorbed through the skin. In case of accidental contact with the skin, the corresponding area should be thoroughly cleaned with soap and water. In case of contact with the eyes, they should be thoroughly rinsed with water. People with known hypersensitivity to carbetocin or any of the excipients should avoid contact with the veterinary medicinal product. Women of childbearing age should administer the product with special caution. 4.6 Adverse reactions (frequency and seriousness) In very rare cases carbetocin may have a uterotonic effect in the late pregnancy.
The frequency of adverse reactions is defined using the following convention: – very common (more than 1 in 10 animals treated displaying adverse reaction(s)) – common (more than 1 but less than 10 animals in 100 animals treated) – uncommon (more than 1 but less than 10 animals in 1,000 animals treated) – rare (more than 1 but less than 10 animals in 10,000 animals treated) – very rare (less than 1 animal in 10,000 animals treated, including isolated reports). 4.7
Use during pregnancy, lactation or lay The veterinary medicinal product is indicated to induce milk ejection. See also 4.3 Contraindications. 4.8 Interaction with other medicinal products and other forms of interaction
The administration of oxytocin after the administration of the veterinary medicinal product is un-necessary. Due to a possible intensification of the effect of oxytocin, undesirable uterine spasms may be induced.
You can download the datasheet here
You can visit the manufacturer’s website here